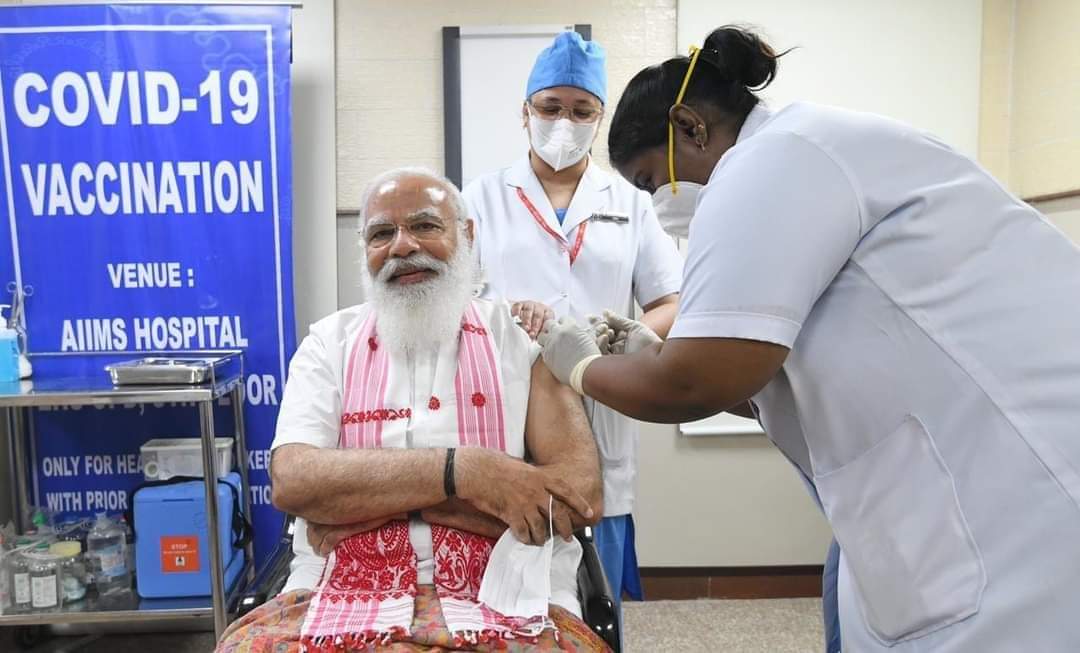
New Delhi: On 28 May 2021, at the height of the deadly second wave of Covid-19 cases, the Modi government announced that it would vaccinate all adults by the end of the year. Six months later, with only 39.7% of adults fully vaccinated, the government is certain to not meet this target, putting the country at greater risk of another wave in a pandemic that has officially taken over 4,60,000 lives.
The failure to vaccinate more Indians is particularly significant because India is one of the few countries in the world to have developed an indigenous public vaccine, which was rolled out in January 2021. The indigenous Covaxin has contributed only about 11% of doses administered, and experts blame the failure to ramp up Covaxin’s production, in particular the government’s strategy of relying exclusively on the small, Hyderabad-based private company Bharat Biotech International Limited, for India's sluggish pace of vaccination.
Despite Covaxin being developed with public resources, the government has offered a virtual monopoly to Bharat Biotech, in return for a 5% royalty on net sales, and a one-time fee of Rs 5,00,000. This indicates that one private company is cornering disproportionate gains from a vaccine developed with public money, experts have told Article 14.
The indigenous vaccine is also one of the most expensive in the world. In India, Covaxin costs more on average than the private Covishield vaccine.
Responses to 10 applications filed by Article 14 under the Right To Information (RTI) Act, 2005, over seven months show that the Modi government has steadfastly kept its memorandum of understanding (MoU) with Bharat Biotech for Covaxin production secret, the royalty receipts schedule vague, and production estimates unclear. This secrecy is apiece with its responses to RTI applications filed by others on multiple aspects of pandemic policies.
Its responses also reveal that the government has received just Rs 33.65 crore in royalty from Bharat Biotech on 30 August 2021. Based on publicly available information on Covaxin sales and orders, it appears that the royalty sum is significantly under-reported.
Article 14 sent detailed queries to Bharat Biotech. The story will be updated if and when it responds.
After Covaxin received an emergency-use-listing on 3 November from WHO, Bharat Biotech is gearing up for exports. In February itself, Bharat Biotech tied up with American pharmaceutical company Ocugen, Inc for regulatory approval and commercial release of Covaxin in the USA. As per a press release, Bharat Biotech will retain 55% of the profits. For a similar deal for the Canadian market, Ocugen made an advance payment of $15 million (about Rs 109 crore) to Bharat Biotech in June.
[[https://tribe.article-14.com/uploads/2021/11-November/16-Tue/ModiVaccine2.jpg]]
Meanwhile, the government is now seeking a $2 billion loan (over Rs 14,956 crore) from the Asian Development Bank and the Asian Infrastructure Investment Bank for procuring vaccines.
Two Parties Jointly Own Rights, One Gets Only 5% Royalties
In a publicity blitz unleashed by the Modi government on 21 October to mark 100 crore vaccine shots, the prime minister claimed that the vaccine was given to Indians free of cost. This is misleading, for citizens have paid for the vaccine either through taxes, or outright purchases in private hospitals (where Covaxin sells at Rs 1,200 to 1,410 per dose).
The MoU with Bharat Biotech also suggests a deal whose financial aspects undermine public interest.
In March 2020, the National Institute of Virology (NIV), an institute of the Indian Council of Medical Research (ICMR), isolated a viral strain for vaccine development. However, instead of investing in public sector manufacturing as in the past, the ICMR tied up in May 2020 with Bharat Biotech for the production and rollout of Covaxin.
By May 2021, as the second wave was surging, experts began to question if Bharat Biotech held exclusive rights to manufacture Covaxin, and if so, why. The ICMR clarified to the media and then to the Supreme Court that the MoU was non-exclusive, and provided for ‘sharing’ the intellectual property rights along with a 5% royalty clause on net sales, to be paid every six months.
In July 2021, a Bharat Biotech official told Business Standard that the company had invested Rs 500 crores in Covaxin development and production.
The ICMR was not forthcoming on the investment of public funds in Covaxin, until the Supreme Court initiated a suo motu public interest litigation in April 2021. In May 2021, the ICMR told the court that it had invested Rs 35 crore in the development of Covaxin.
It was only in July, in response to a question in the Rajya Sabha by the leader of the opposition and Congress member of parliament, Mallikarjun Kharge, that the government revealed that the intellectual property in Covaxin was in fact jointly owned with Bharat Biotech, and that it had transferred the viral strain isolated by the NIV to Bharat Biotech for a one-time fee of Rs 5 lakh.
These disclosures led many experts and activists to criticise the ICMR for claiming only 5% as royalty.
Economist and professor R Ramakumar of the Tata Institute of Social Sciences (TISS) told Article 14: “The royalty of 5% is an anomaly in the contract. When IPR is jointly owned by two institutions, it makes no sense that one of the parties gets 95% and the other only 5%.”
He said in terms of investment, Covaxin is the cheapest vaccine in the world. “Yet it is one of the most expensive in the market,” he said, “and the government has allowed one company to usurp the benefits.”
In her reply to Kharge, minister of state for health & family welfare Dr Bharti Pravin Pawar claimed that the royalty of 5% was decided based on benchmarking royalty percentages domestically and internationally, by organisations such as the World Health Organisation (WHO) and the United Nations Development Programme (UNDP) .
Neeta Sanghi, an industry veteran with over three decades’ experience in managing pharmaceutical supply chains, told Article 14 that the WHO and UNDP benchmarks referred to royalty in cases of compulsory licensing, generally issued by governments to reduce prices and improve access.
“In this case, the government has used the royalty arrangement to enable a monopoly. Whether these benchmarks apply is questionable,” said Sanghi.
On 3 June, Article 14 filed an RTI request with ICMR asking for:
1. Royalty payments received by ICMR from the sales of Covaxin between 1 January 2021 and 31 May 2021; and
2. Month-wise sales of Covaxin doses between 1 January 2021 and 31 May 2021.
As the ICMR did not reply within the statutory time limit of 30 days, Article 14 filed an appeal on 6 July. On 18 August, the ICMR replied, stating that “...we have not received any royalty from M/S Bharat Biotech till date. However matter (sic) is under process....”
In effect, seven months after its release, the public exchequer had not received even a rupee’s return on Covaxin sales.
On 18 October, in response to yet another RTI application from us, the ICMR said it had received Rs 33.65 crore as royalty on 30 August. Since 5% of sales were due as royalty every six months, this means that Bharat Biotech claimed to earn Rs 673 crore until June 2021 from Covaxin.
Stonewalling Requests For Information, Underreporting Revenue
So far, the ICMR has refused to reveal crucial data such as the number of doses and time period corresponding to the royalty receipt of Rs 33.65 crore. (Article 14 has filed an appeal contesting the response.)
Their refusal to place this information in the public domain makes it difficult to definitively scrutinise the royalty amount.
However, Rs 673 crore in Covaxin sales revenue seems to be a clear case of underreporting. By 30 June, 4.03 crore Covaxin doses had been administered in India as per the CoWin dashboard. The government disclosed partial information on orders and prices under RTI to transparency activist Commodore Lokesh Batra (retired), and to the Supreme Court in its affidavit dated 26 June.
Based on this, a back-of-the-envelope calculation indicates that Bharat Biotech likely earned at least Rs 1,010 crore in the first six months, as Table 1 shows.
* Assuming a conservative average price of 1000 rupees a dose. In May, there were reports of Covaxin selling at up to Rs 1,500 per dose, and more in the black market. After criticism from the Supreme Court, the Union government, starting June 21, capped the price at Rs 1,200 per dose for Covaxin, exclusive of taxes and hospital fees.
If we conservatively assume that Bharat Biotech sold the balance 28 lakh doses (out of 4.03 crores administered by 30 June) to the Union government at the regulated price of Rs 150 per dose, it would have earned an additional 42 crores, taking its total earning in the first six months to about Rs 1,010 crores.
Further, in April, Bharat Biotech also announced Covaxin exports at Rs 1,123 - 1,498 per dose, and as per the Union of India’s affidavit dated 26 June to the Supreme Court, in private hospitals of Nepal, Covaxin was selling at $35 per dose (over Rs 2,000 a dose).
It therefore appears that Bharat Biotech earned much more than Rs 673 crores in the first six months, and owed ICMR a royalty higher than Rs 33.65 crores.
The government has furthered its financial opacity by claiming in response to another RTI request filed in August by Batra that it does not have data on how many vaccine doses are administered by private hospitals and health service providers. This contention is false because it was itself facilitating supplies to private hospitals and it collects real-time vaccination data via its CoWin dashboard. It therefore must know the break-up of doses administered in the country.
Public Money Invested, But MoU Not Disclosed
On 27 April, Article 14 filed RTI applications with both the ICMR and the NIV, seeking a copy of the MoU with Bharat Biotech, and the amount and detailed break-up of funds invested by the respective organisations in the pre-clinical and clinical development of Covaxin.
Dr Nivedita Gupta of the ICMR denied the entire request that very day. Gupta did not justify the denial by invoking any exemption clause as required by the RTI Act, instead offering this logic, “The information is not available in the public domain.”
Her response also went against section 4 of the RTI Act that mandates suo motu disclosure of information, and the office memorandum No 1/6/2011-IR dated 10 July 2020 from the department of personnel & training that specifically called for suo motu disclosures for matters related to Covid-relief.
On 11 May, Dr Paresh Shah of the NIV too denied our request, stating:
“As per the ‘Confidentiality’ clause of the Memorandum of Understanding (MoU) between ICMR-NIV and Bharat Biotech International Limited, it has been expressly agreed by both the parties to maintain confidentiality about all the information….”
Dr Samiran Panda, the first appellate authority of the ICMR, did not hear Article 14’s first appeal within the statutory period of 30 days. At the NIV, on 24 June, Dr Priya Abraham, the director, rejected the appeal, stating:
“The disclosure of information can have major commercial implications. Hence, This information cannot be disclosed U/S 8(1)(d) of the RTI Act 2005. In this connection, you may please refer CIC decision in Second Appeal No. CIC/ICOMR/A/2021/618110 in the matter of Ms. Apurva Singh Vs. Indian Council of Medical Research…”
The reference here was to a second appeal hearing on 3 June in which the chief information commissioner (CIC) Y K Sinha had ruled against disclosure of the MoU. At the hearing, Dr R Lakshminarayanan, the deputy director general (administration) of the ICMR, argued that:
“the document sought is not a public document and is a highly sensitive agreement between the ICMR and Bharat Biotech having commercial implications and can be used by other competing pharmaceutical companies for their commercial interests. Thus, the information sought is exempted u/s 8 (1) (d) of the RTI Act.”
On 22 June, Article 14 filed a second appeal against the ICMR, which the chief information commissioner, Y K Sinha, heard on 15 September.
By the time of this hearing, the Union health ministry had disclosed some details of the MoU, such as the aforementioned royalty obligations, the obligation to pay a one-time fee of Rs 5,00,000, etc., to the Parliament, in its reply to Kharge on 20 July.
In the reply, the ministry also admitted that other companies had not been permitted to develop Covaxin. This contradicted the ICMR’s and NIV’s contention to RTI applicants and the CIC that transparency around the MoU would harm Bharat Biotech’s market position and aid its competitors.
At the 15 September hearing, Article 14 highlighted these discrepancies to the CIC. We pointed out that Covaxin production was falling short of targets, and that despite its origins in a public-private partnership, it was one of the most expensive vaccines in the world. We argued that making the MoU and details of public investment in the vaccine public would allow citizens to offer constructive feedback to the government.
Dr Jerin Jose Cherian and Dr Tanu Anand representing the ICMR at the hearing did not offer any counter-arguments.
On 17 September, CIC Sinha called Gupta’s reply to the RTI request “evasive and untenable,” and cautioned her against blanket and generic denials. However, he did not order disclosure, and moreover did not even pass an order. Instead, he sent the matter back to ICMR’s first appellate authority Panda, to convene a hearing and to issue a reasoned, speaking order by 15 November.
Shailesh Gandhi, a former central information commissioner, said the CIC not passing an order was “completely unacceptable.” Gandhi told Article 14 that the CIC should have reprimanded the ICMR’s appellate authority for not hearing the matter.
“Given that the ICMR officials did not advance any arguments during the hearing (for keeping the MoU secret), there was no reason to send it back,” said Gandhi. “The citizen is expected to get the information.”
Despite our reminder to the ICMR, Panda has flouted the CIC’s order of holding a hearing and passing a reasoned decision on the matter by 15 November. The government’s MoU with Bharat Biotech and details of public funds invested in Covaxin’s development remain secret.
This despite the Central Information Commission and court rulings (here, here and here) and the Comptroller & Auditor General (CAG) laying down that MoUs should be public documents, saying every citizen is entitled to know the terms on which governments enter into agreements on their behalf and with public money.
Ramakumar is among a number of academics, activists, journalists, lawyers and others (here and here) who have called upon the government to make the Covaxin MoU with Bharat Biotech public.
Ramakumar told Article 14 the government could have redacted parts dealing with commercial confidence and disclosed the MoU. “Its refusal to do so makes it appear as if either the government or Bharat Biotech does not want to reveal the exact terms of the contract,” he said.
If the average cost of producing a dose is Rs 30-Rs 80, as reported, then even at the regulated price of Rs 150 per dose, the company was making “a killer of a profit”, said Ramakumar.
The sales figure of Rs 673 crore in the first six months is a huge windfall for a small company like Bharat Biotech, Ramakumar said. To put this in perspective, the company’s revenues across all product lines in the financial year 2019-20 was Rs 1,080 crores, the Business Standard reported. The company has likely already recouped a significant amount of its investment in Covaxin development & production.
Ramakumar also observed, “the fact that they have an effective monopoly over a vital public good in a pandemic since May 2020 is yet another shocking detail.” He said this was a case in which the CAG should intervene.
Sanghi, the pharma industry expert, also pointed to the irony of the government fighting internationally since August 2020 for a waiver of intellectual property rights in Covid-19 vaccines, while on the other hand creating a monopoly at home. “Such secrecy is definitely not justified,” she said. “The MoU should be a public document because the government is accountable to us citizens.”
No Clarity On Bharat Biotech’s Production Capacity
Ever since the vaccine shortages of the second wave, the Modi government has offered widely varying projections of Covaxin supplies, as summarized in Table 2.
There is no clarity, even in November, on Bharat Biotech’s actual production capacity. It was manufacturing only about 60 lakh doses per month in March. In its affidavit dated 9 May to the Supreme Court, the government declared Bharat Biotech’s capacity to be 2 crore doses per month. However, by 30 August, actual supplies were 8 crore doses or 1 crore doses per month, on average.
On 16 April, the ministry of science and technology had announced that it had identified three public sector undertakings—Bharat Immunologicals and Biologicals Limited (BIBCOL), Indian Immunologicals Limited (ILL), and Haffkine Biopharmaceutical Corporation Limited—and sanctioned Rs 30 crore, Rs 60 crore and Rs 65 crore respectively for the necessary upgradation to manufacture Covaxin.
All three PSUs, the ministry declared, were to begin supplying doses by October—though Haffkine had asked for a year’s time. As of November, Bharat Biotech remains the only supplier of Covaxin.
On 8 June, Article 14 filed an RTI request with BIBCOL asking for a copy of the MoU for Covaxin production, budgetary estimates for upgrading existing facilities, and the amount of money received from the Union government.
On 5 August, BIBCOL replied, stating: “no information is available.” Only on 14 August did BIBCOL advertise a tender notice inviting bids to set up the necessary infrastructure for Covaxin production. Bids were due 15 September, and BIBCOL requires the contractor to complete the project four months after awarding the tender.
On 5 August, Article 14 also filed an RTI request with the National Dairy Development Board, which established ILL, seeking similar details. On 3 September, the ILL denied the request stating that it was not subject to the RTI Act, and that information could not be shared as disclosure might harm the competitive position of a third party.
On 13 August, the ministry of science and technology announced that ILL had begun supplying “Covaxin drug substance”, i.e. the raw material for the vaccine, to Bharat Biotech. Whether and when it would begin supplying the vaccine independently remains unclear.
Given that manufacturing and testing processes for Covaxin take 90-120 days, the other two PSUs are unlikely to begin supplying doses until some time in 2022.
Article 14 sent detailed queries to BIBCOL, ILL and Haffkine. The article will be updated if and when they respond.
People’s Vaccine Frittered Away
Speaking to Article 14, Ramakumar said that had India rapidly scaled up Covaxin production, more people in the world could have been vaccinated—only about 4.5% of the population in low-income countries has been vaccinated so far. “India could have been a leader in vaccinating the world, while providing the government and private companies some profit,” he said. “It’s unfortunate for the people’s vaccine that this opportunity was lost.”
Sanghi told Article 14 that while Bharat Biotech’s low capacity was well-known, the government could have brought together all or most of India’s vaccine manufacturers for Covaxin production and facilitated capacity upgradation in 2020 itself.
“When the Russian Direct Investment Fund could tie up in March and April 2021 with six manufacturers in India, for a combined annual capacity of 80 crore doses of Sputnik V, it is inexplicable that the government did not tap all the vaccine manufacturing capacity in the country for Covaxin (a point also made by the noted immunologist Satyajit Rath in a June 2021 interview to Article 14).”
Sanghi said that Bharat Biotech’s effective monopoly may not end even when the PSUs begin production; since their agreements with Bharat Biotech are not in the public domain, we do not know whether the PSUs will be subcontractors or independent manufacturers.
“The government has been extremely short-sighted and has frittered away the possibility of being truly aatmanirbhar (self-reliant),” said Sanghi. “Business schools should teach India’s vaccine procurement program as a case study in how not to manage a supply chain during a pandemic.”
A review of Covid-19 vaccine development in the West, published in Fortune, found companies collecting billions in government grants, hoarding patents, disclosing little about their deals, and charging premium prices. In the low-cost ‘pharmacy of the world’, a similar trajectory has emerged.
The Modi government has kept critical information hidden from public scrutiny, despite having invested Rs 100 crore of public money in the vaccine—along with ICMR’s Rs 35 crore rupees, the government announced a grant of Rs 65 crore to Bharat Biotech in April, and committing thousands of crores worth of orders, thus guaranteeing revenues.
Public health experts, activists, Parliamentarians and the courts have for months sought greater clarity and transparency from the government. The government has however stonewalled scrutiny by steadfastly denying RTI requests, in violation of the law.
On 31 May, as the second wave devastated India, Justices Dhananjaya Chandrachud, L Nageswara Rao and S Ravindra Bhat ordered the government to place “all the relevant documents and file notings reflecting its thinking and culminating in the vaccination policy…” before their bench within two weeks.
Despite the provision for suo motu disclosures under the RTI Act, the justices did not order that the same information be made public.
In any case, the government ignored the court’s direction in its affidavit filed on 26 June. The bench has passed no orders since.
(Aniket Aga teaches environmental studies and anthropology. He is the author of Genetically Modified Democracy: Transgenic crops in contemporary India published by Yale University Press. Chitrangada Choudhury is a journalist and on the editorial board of Article 14.)